The questions that drive us
Research Focus
We study how the immune system shapes the brain across development and disease. Our work combines single-cell genomics, enhancer biology, and in vivo models to understand microglial function, ontogeny, and dysfunction in glioblastoma and neurodevelopmental disorders.
Our approach bridges molecular neuroscience, immunology, and computational biology, aiming to identify therapeutic strategies through a deeper understanding of gene regulation in brain-resident immune cells.
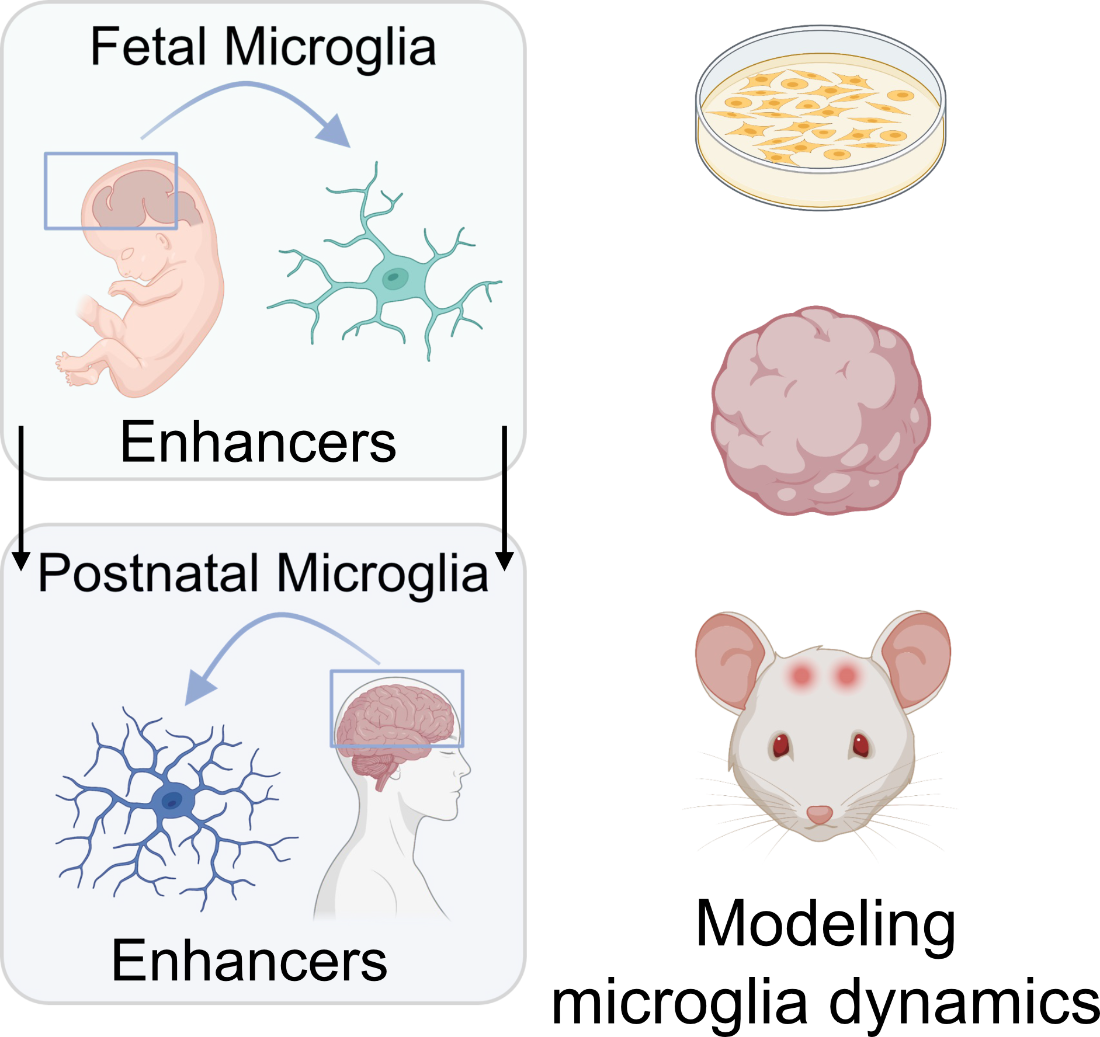
Transcription regulation of microglia
Comparison of the transcriptomic and epigenomic profile of human microglia through development allowed us to predict gene regulatory networks unique to each developmental stage. Additionally, we also found several genes and transcription factors implicated in neurodevelopmental and neurodegenerative disorders that are highly expressed in microglia. Utilizing both genetic mouse models and stem-cell based systems, we seek to investigate the specific gene programs regulated by these transcription factors and how these programs are disrupted in neuropathology.
Mechanisms of cell “memory”
Throughout development, from embryonic to old age, humans and animals are exposed to a variety of environmental insults. The timing, type, strength, etc of the insult have been suggested, epidemiologically, to increase risk for neurodevelopmental, neuropsychiatric or neurodegenerative disorders. Recent studies have suggested that memory of these environmental disturbances are encoded in the genome and can affect gene expression patterns later in life. We are interested in elucidating the gene regulatory networks activated or dampened that are shared and distinct between major cell types of the brain as a result of environmental perturbation at critical developmental timepoints.
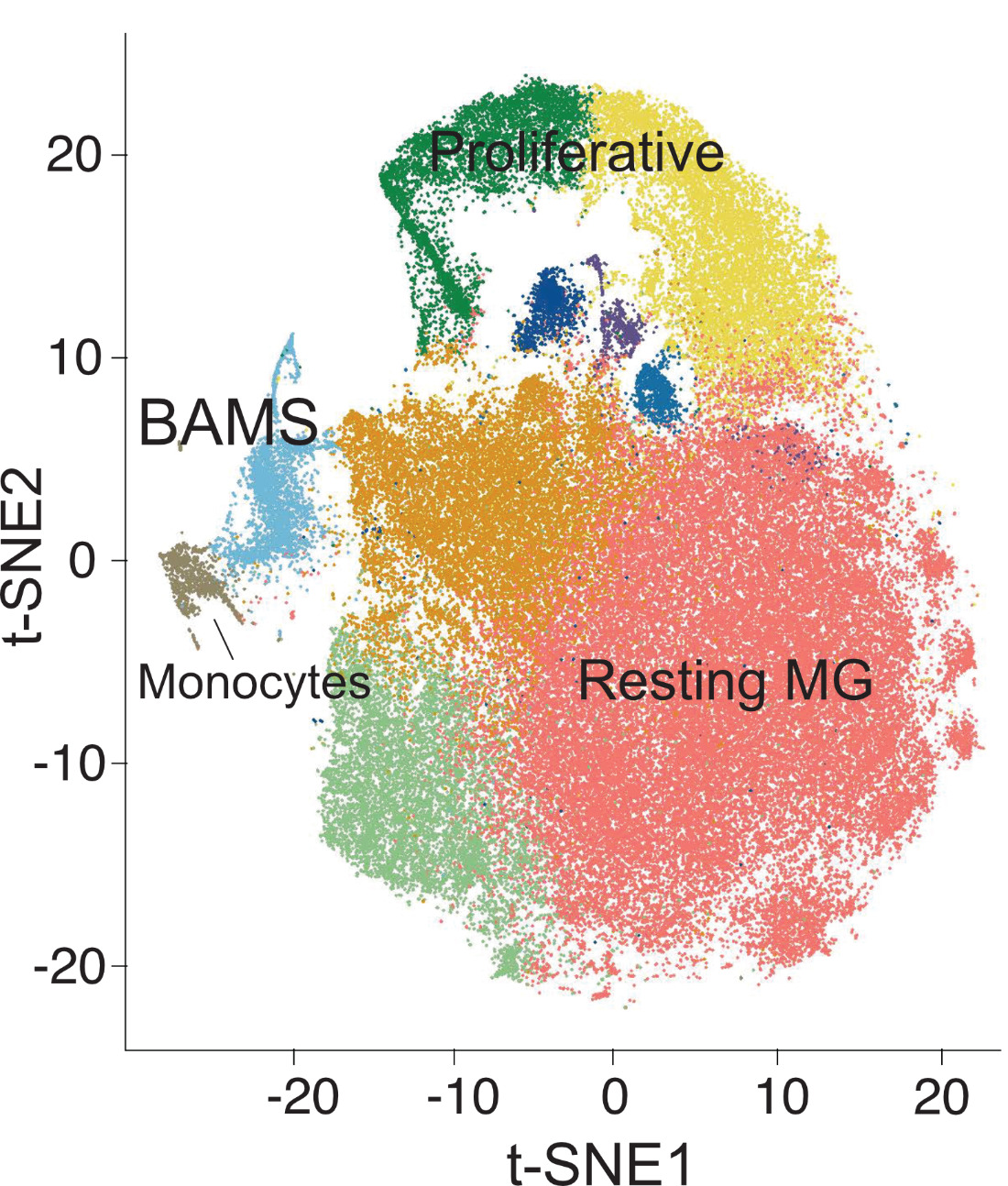
Determinants of brain macrophage types
In homeostatic conditions, yolk-sac derived microglia and border associated macrophages (BAMs) are the macrophages of the central nervous system. In aging, AD, or parasitic infection, recruited monocytes rapidly enter the brain and differentiate into macrophages. ScRNAseq studies in mice and humans, under homeostatic and diseased conditions, have shown that parenchymal microglia, BAMs, and recruited monocytes are transcriptionally distinct. However, these different populations have diverse functions in infection and neurodegeneration. Our goal is to determine the enhancer repertoire and the transcriptional machinery driving the functional differences between these populations in homeostasis and in aging.